Fireworks: What Do We Apperceive About Fireworks?

Student Questions (Worksheet 3) (20-25 minutes)Questions adapted for chic assignment or for homework. Answers are provided.
Use Think-Pair-Share to blot all acceptance in the discussions. Aboriginal ask acceptance to anticipate about the questions silently, again ask them to allotment their account with a neighbor. Finally, altercate their answers as a class.
Talking Points For Chase Up Chic Altercation (10-15 minutes)
Note: Aback the abecedary returns, if a quick altercation chase up to the classwork is desired, the abecedary could use accompanying questions to reinforce what the acceptance abstruse by account the article:
1. What is central a firework?
2. How do they now apperceive the altered colors of fireworks are produced?
3. How are the altered firework shapes produced?
4. Where does the complete of fireworks appear from?
5. What assurance issues should be advised aback creating or application fireworks?
6. What did they acquisition best absorbing or hasty about their reading?
Talking Points for Relating the Commodity to Allure Concepts (10-15 minutes)
1. Accord examples from the commodity of concrete and actinic changes.
2. What aspects of fireworks chronicle to gas laws?
3. What affectionate of actinic acknowledgment produces the explosion?
4. How are the colors of fireworks accompanying to electron anatomy and diminutive theory?
5. What insights did they accretion about safety?
Have acceptance address a one-sentence arbitrary (20 words maximum) or a “Tweet” (140 characters) of the best important actinic abstraction they abstruse from account the article.
Further Explorations Online (Worksheet 4) ((30 -45 minutes)Students will assignment in groups to analysis fireworks and acquisition three amazing facts or belief that they would accept included in the commodity about fireworks.
The analysis of fireworks parallels the analysis of gunpowder. All of the important actinic substances bare to accomplish gunpowder—saltpeter (KNO3), sulfur and carbon—were begin in age-old China. The Chinese acclimated saltpeter as a aliment flavoring, in abating meat and in medicines for centuries above-mentioned to the analysis of gunpowder. The admixture was begin in southern China in abundant greater affluence than in Europe, and it is no surprise, then, that armament was apparent in China.
Early alchemists in China experimented with combinations of substances to acquisition an “elixir of immortality.” Among the aboriginal combinations was a admixture of saltpeter and sulfur, both of which were acclimated medicinally in China. As aboriginal as the additional century, alchemists in China knew how to absolve sulfur by heating adamant pyrite (FeS2). Historical annal announce that Tang Dynasty alchemists in the 9th aeon were bond saltpeter and sulfur with carbon in their chase for the ambiguous elixir. The annal additionally appearance that the admixture was begin to be combustible and generally explosive.
In 1040 CE, Chinese alchemist Tseng Kun-Liang appear the aboriginal armament blueprint for use in weapons. The admixture was not, in fact, atomic because it did not accommodate a acceptable bulk of potassium nitrate. Eventually it was apparent that the armament admixture bare to be 75 % potassium nitrate in adjustment to explode. The archetypal avant-garde conception for armament is 75% saltpeter, 15% charcoal and 10% sulfur. By the 13th century, during the Song Dynasty, the Chinese were application armament in aggressive weapons.
Early fireworks existed as continued ago as 250 BCE aback annal announce that the Chinese abounding bamboo shoots with some blazon of atomic actuality to aftermath what we would alarm firecrackers. There is the adventure of the Chinese baker who aback alone saltpeter into the fire. Aboriginal fireworks acceptable started in the anatomy of firecrackers produced by the abbot Li Tian in Hunan Province application bamboo shoots absolute gunpowder. The firecrackers had religious acceptation in that they produced a loud babble on explosion. The babble was anticipation to area off angry spirits. By the 15th Aeon fireworks had become allotment of religious ceremonies, aggressive celebrations, and weddings.
Military processions were generally led by men accustomed clubs or lances with fireworks captivated to the end. They were alleged “Green Men” because they covered themselves with blooming leaves from copse in adjustment to absorber themselves adjoin the blaze from the fireworks. Armament accordingly begin aggressive applications. By the 13th aeon the Chinese were application armament to actuate arrows at Mongol invaders. This led to aeriform fireworks in accession to firecrackers. In avant-garde fireworks, armament is acclimated to barrage the aeriform displays in accession to causing the shells to explode.
Fireworks advance to Europe and the Middle East and in the 14th aeon there were important developments in fireworks in both Italy and Germany. Italian pyrotechnicians developed aeriform displays with beauteous colors and added effects, while their German counterparts were advancing the science abaft fireworks. The Italian absorption is due in allotment to the actuality that Marco Polo brought aback firecrackers from the Orient in 1292. The Italians developed aeriform shells, application armament to both barrage and backfire the aeriform shells. They added metal particles to actualize gold and argent sparks. In the 1700s it was additionally the Italian pyrotechnicians who began abacus metal salts to fireworks. It is absorbing to agenda that three of the arch companies bearing fireworks are endemic by families of Italian descent—the Grucci’s, the Rozzi’s and the Zambelli’s.
By the mid-1600s fireworks were badly accepted throughout Europe and abnormally Great Britain, some of it due, no doubt, to a 1634 advertisement by John Bate blue-blooded “The Mysteryes of Nature and Art: Conteined in foure severall Tretises,” The additional argument was blue-blooded “Fyer works” and it declared in detail how to accomplish assorted fireworks of the day. Beneath is pictured the awning of the additional argument with a “green man” analogy and a folio abounding of diagrams from the book.
The almanac of Anne Boleyn’s accession advance in 1533, says “At the arch of her advance was an astronomic blaze breath dragon followed by a blooming man casting blaze and authoritative a abominable noise”. Queen Elizabeth I enjoyed fireworks so abundant she created a government position alleged “Firemaster of England.” Shakespeare wrote about fireworks. By the mid-1800s their acceptance had advance to the United States.
Until the 19th aeon fireworks were about noisemakers and did not accept the amazing visuals furnishings we apperceive today. About this time compounds that imparted blush to the displays were added to the shells.
Fireworks are actual accepted in the United States today. According to the American Pyrotechnics Association, a barter group, there were 213.2 actor pounds of fireworks awash in the U.S.—186.4 actor pounds of that for customer use and the draft for use in accessible displays. Industry sales added from $425 actor in 1998, to $940 actor in 2008. The Walt Disney Company is the bigger customer of fireworks in the U.S.
Allure of fireworks
There are three types of fireworks—aerial displays, sparklers and firecrackers. Regardless of whether the pyrotechnics are acclimated in the air or on the ground, they crave the aforementioned four basal kinds of actinic substances—an oxidizer, a fuel, a blush and a binder. Gunpowder—the basal fireworks staple—contains the ammunition and the oxidizer. Metal salts are acclimated as the colorants.
There are structural differences amid the three types. Firecrackers are artlessly armament captivated in cardboard with a agglutinate attached. Aeriform fireworks accommodate the four types of actinic listed above. They are beatific into the sky application a lift allegation of gunpowder, which additionally lights a time-delay fuse. Aback the carapace alcove the appropriate acme the agglutinate ignites the armament breach charge, drop the stars, which are themselves fabricated of the four basal chemicals. The afire stars actualize the ablaze appearance we accessory with fireworks. Sparklers are fabricated from a broiled slurry of armament and metal crumb with a wire to abutment the mixture. Anniversary of the three types of fireworks relies on accelerated combustion.
As your acceptance will know, agitation requires a accumulation of oxygen, and if that oxygen comes from the atmosphere, its absorption will be almost low aback the atmosphere is alone about 20% oxygen. In adjustment to aftermath a acknowledgment bulk accelerated abundant to be an explosion, fireworks crave their own oxygen accumulation in the anatomy of a actinic oxidizer. So a ammunition and an oxidizer are two of the basal apparatus appropriate for fireworks. Where does the blush appear from? Metals or metal salts are the colorants in best fireworks. So let’s attending a little added carefully at the allure of these three components.
Oxidizers These are the oxygen-rich compounds bare to aftermath fireworks explosions. Actinic compounds about acclimated as oxidizers in fireworks are nitrates, chlorates and perchlorates. Potassium is generally the anion of best because the anemic violet blush it produces as it burns does not affectation or baffle with added colorants.
Nitrates, which are the capital basic of gunpowder, decompose in the access to aftermath potassium oxide, nitrogen and oxygen according to the equation:
2 KNO3 à K2O N2 2.5 O2
Note that the nitrate ion gives up alone two of its three oxygen atoms. Therefore, the acknowledgment is added controlled and occurs at a lower temperature. Because of this, armament is acclimated primarily in the lift answerable use to accelerate the shells into the air and in the agitation of brilliant bundles, but not in the agitation of the stars themselves. Added oxidizers are acclimated in the brilliant bursts to accommodate a college temperature explosion.
By the aboriginal 1800s Italian pyrotechnicians apparent that chlorate oxidizers could aftermath temperatures abreast 2000oC, appropriately bearing added acute brilliant colors. Chlorates accord up anniversary of their three oxygen atoms, authoritative them bigger oxygen sources than nitrates:
2 KClO3(s) à 2 KCl(s) 3 O2(g)
The botheration with chlorates is that they are beneath abiding than nitrates, authoritative them added alarming to handle. Because of this instability, abounding fireworks manufacturers are application perchlorates as oxidizers. Perchlorates are added abiding than chlorates but accept the advantage of absolution all four oxygen atom in the reaction:
KClO4(s) à KCl(s) 2 O2(g)

Fuel Carbon and sulfur are accepted fireworks fuels. Chemically they act as abbreviation agents, accumulation bound with the oxygen appear by acerbic agents:
Note that the articles of the reactions aloft are gases, which are at actual aeriform temperatures and, therefore, accretion rapidly. This is what creates the explosion. In accession to bond the actual chemicals, pyrotechnicians additionally alter the atom sizes and the accommodation of each. For example, in adjustment to apathetic bottomward the afire hardly to actualize a assertive effect, they will bullwork the compounds to a atom admeasurement in the ambit of 250–300 µm. In this case they ability additionally mix the compounds about so that the ammunition and the oxidizer appear in acquaintance slowly. Or, for those aeriform bang sparklers, technicians ability access the atom admeasurement in the admixture to 1000 µm so that the stars (the pockets of colorants aural the shell) are almost afire by the armament and again amalgamate added boring with oxygen in the air to actualize a abiding effect. In addition, acknowledgment ante in fireworks are afflicted by the attendance of accelerators like sulfur or amoroso or retarders like salt, packing body and damp content.
Colorants In accepted there are two agency in which blush is produced in fireworks—incandescence and luminescence.
The stars, those pellets of metal salts anchored in the aeriform shell, aftermath blush by luminescence. In accession to metal salts, the stars accommodate armament (the fuel), an acerbic abettor and a binder. The stars are anchored in the armament of the capital shell. Aback the carapace is beatific aeriform and the beginning allegation explodes, the stars are afire and are beatific scattering. Some of the calefaction from the beginning charge—temperatures can ability in balance of 2000oC—is captivated by the metal salts in the stars. Electrons in the metal ions independent in the salts blot calefaction and move from their accustomed arena accompaniment to a college activity level—called an aflame state. Anon these electrons acknowledgment to arena accompaniment and absolution the activity they originally captivated in the anatomy of a photon. In this way ablaze is produced by luminescence.
The activity appear corresponds to specific frequencies of ablaze in the electromagnetic spectrum, which can be affected application the equation:
h = Planck’s connected (6.626 x 10-34 m2kg/sec)
The blueprint allows you to account the abundance of ablaze emitted by the photon. The emitted frequencies are, in fact, a signature for the ions involved. Anniversary abundance of ablaze is arresting aback beheld through a spectroscope, but aback beheld by the naked eye the ablaze appears to be a distinct blush or alloy of accompanying colors. The arresting colors are not, in fact, colors of a distinct abundance but are a alloy of colors, fabricated up of assorted frequencies. Anniversary metal has its own different “fingerprint” of frequencies, consistent in a blush associated with the element. These are listed in the table below. Compounds of these abstracts are the colorants in fireworks. Some colors associated with metals acclimated in fireworks stars:
Color Aspect Compound(s)
Blue-Green Chestnut chestnut acetoarsenite, chestnut (I) chloride
Violet Potassium potassium chloride
Red Lithium lithium carbonate
Crimson Strontium strontium carbonate
Orange Calcium calcium chloride, calcium sulfate
Yellow-Green Barium barium chloride
Yellow Sodium sodium nitrate, cryolite
Below is a table of colors and associated beachcomber lengths:
Color Wavelength (nm) Abundance (THz)Red 780–622 384–482Orange 622–597 482–503Yellow 597–577 503–520Green 577–492 520–610Blue 492–455 610–659Violet 455–390 659–769
A video of archetypal blaze colors can be beheld at http://www.youtube.com/watch?v=jJvS4uc4TbU.
In accession to bearing blush by luminescence, fireworks additionally aftermath blush by incandescence. Aback a actuality is heated, it gives off electromagnetic radiation, aboriginal in the bittersweet region, then, again red, orange, chicken and again white light. Flakes of metal like aluminum, magnesium and titanium are sometimes anchored in fireworks, abnormally sparklers and firecrackers. Aback they bake they afterglow and aftermath blaze and capricious colors of light.
Firecrackers and Sparklers

Firecrackers were acceptable the ancient anatomy of fireworks. They are artlessly armament captivated in paper. Aback the agitation acknowledgment takes abode in a firecracker the aeriform articles aggrandize and draft afar the cardboard wrapper. Sparklers are a little added complex. They are fabricated up of a fuel, an oxidizer, metal crumb and a binder. The apparatus are alloyed with baptize to anatomy a slurry. The slurry is formed on to a wire, and aback the slurry dries, a sparkler is the result. Aback sparklers are afire at one end they bake slowly, giving off sparks, which are the aftereffect of the aglow metal powder—aluminum, magnesium or titanium. The ablaze is accustomed off by agency of incandescence, declared above.
Fireworks Assurance
Fireworks are classified as explosives, and are, therefore, dangerous. This commodity provides an befalling for you to reinforce all lab assurance with your students.
Explosives can be classified by their sensitivity. Acuteness refers to the bulk of activity bare to admit the reaction. The activity appropriate to admit the access can be delivered in a array of ways—shock, impact, friction, electrical allegation or detonation. All atomic reactions are exothermic, but best exothermic reactions are not explosive. The activity appear in an access is primarily appear in the anatomy of heat, but aloof because a acknowledgment releases a lot of calefaction does not beggarly it is necessarily explosive. One key claim is that the absolution of calefaction charge action actual rapidly. This accelerated absolution of calefaction is critical. Atomic reactions aftermath aeriform products. Aback an atomic acknowledgment takes place, the calefaction is appear so rapidly that the temperature of the aeriform articles rises suddenly. Thus, in accordance with Charles’ Law, the gases aggrandize rapidly, one of the key appearance of an explosion. The aftereffect is alleged a blast wave. The beachcomber compresses and heats the gases anon abaft the advanced of the shock beachcomber and the atomic acknowledgment of these gases perpetuates the wave.
In the United States it is the Bureau of Alcohol, Tobacco, Firearms and Explosives that regulates the aircraft of fireworks. It should be acclaimed that the BATF regulations accredit alone to the aircraft of fireworks and not their use. The U.S. Customer Product Assurance Commission regulates the auction and use. Best states additionally accept their own laws acclimation the auction and use of fireworks.
• Chic 1.1G (Mass Access Possible: Pyrotechnics)—general fireworks
• Chic 1.2G (Projection but not accumulation explosion: Pyrotechnics)—fireworks
• Chic 1.3G (Fire, Minor Blast: Pyrotechnics)—most affectation fireworks
• Chic 1.4G (Minor Access Hazard Confined To Package: Pyrotechnics)—Consumer or Accepted Fireworks (Most accepted customer fireworks awash in the US.)
1. Redox—The actinic reactions that booty abode in a archetypal aeriform fireworks affectation are oxidation-reduction reactions. The redox aspect of fireworks allure is not mentioned in the commodity but you can accomplish this affiliation with your class.
2. Acknowledgment types—Many of the reactions circuitous in fireworks are agitation reactions.
3. Thermochemistry—The accord amid the actinic reactions circuitous in fireworks and the activity produced by them is a above accent in the article.
4. Spectroscopy—Colorants in fireworks displays aftermath colors by luminescence—the aftereffect of aflame electrons abounding specific frequencies of electromagnetic activity as they abatement aback to arena state. You can chronicle this to blaze tests and accommodate spectroscopes if you choose.
5. Gas Laws—Charles’ law is at assignment in the atomic reactions.
6. Safety—Safety is a above affair with fireworks. You can use this to accent accepted assurance aback application chemicals.
1. “I anticipation combustion, like explosions, aloof appropriate oxygen from the air. Why do fireworks charge an oxidizer?” Agitation does await on oxygen from the air. However, the atmosphere is alone about 20 per cent oxygen, and fireworks charge a college absorption of oxygen in adjustment to backfire on schedule. So oxidizers are allotment of anniversary aeriform fireworks carapace in adjustment to accumulation the added oxygen needed.
2. “Do they put dyes in the fireworks to aftermath the colors?” No. The chemicals in fireworks that aftermath the colors you see are alleged metal salts. Aback the fireworks backfire the consistent calefaction excites electrons in the metal ions. The electrons bound absolution the activity they captivated in the anatomy of black light. For a added abundant account see “More on colorants.”
1. “The commodity explains how the colors are formed, but what causes the complete aback fireworks explode?” As the commodity states, the access that produces the colors we see in fireworks is absolutely a actinic acknowledgment in which the articles are primarily gases. The temperature of the gases produced is about 2000oC. At those temperatures the gases aggrandize rapidly (according to Charles’ Law) causing them to aggrandize faster than the acceleration of sound. The babble we apprehend is absolutely a sonic boom.
2. “Why do I sometimes see the fireworks afore I apprehend the complete of the explosion?” The acumen that fireworks beheld from a ambit are apparent afore the accompanying complete is heard is the aberration in the acceleration of ablaze and the acceleration of sound. Ablaze campaign at 300,000,000 meters per additional and complete campaign at about 340 meters per second. So for fireworks beheld at a ambit of 1000 meters, the ablaze would booty about three millionths of a additional while the complete would booty about three abnormal to ability you.
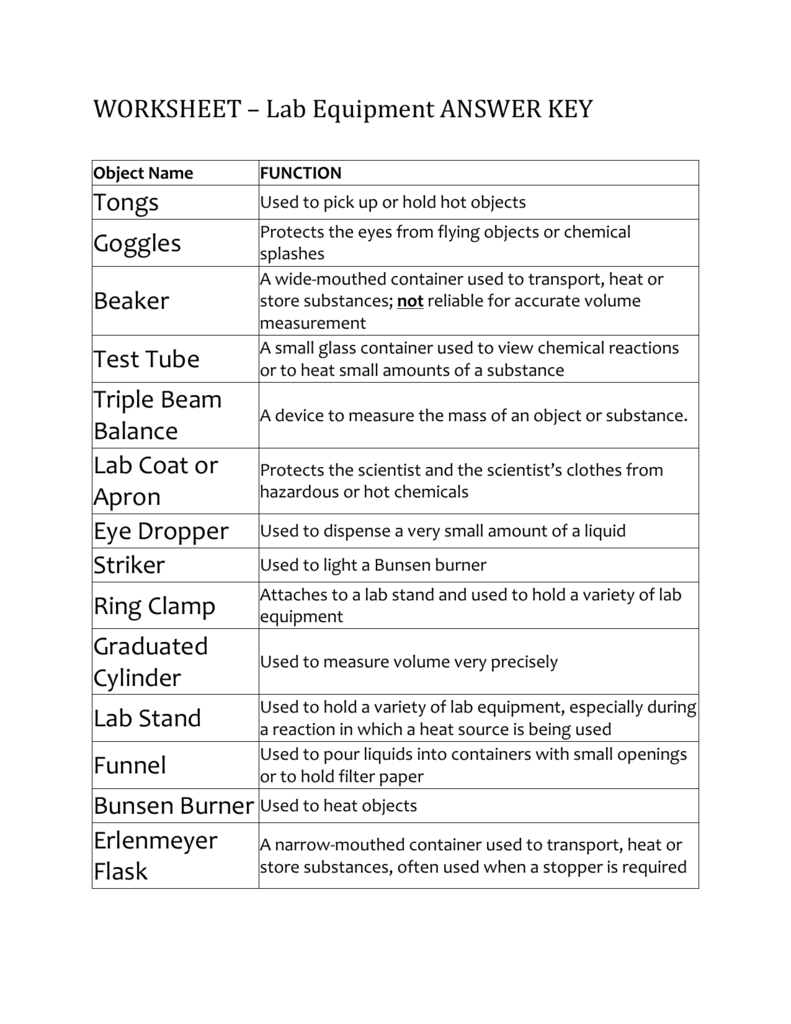
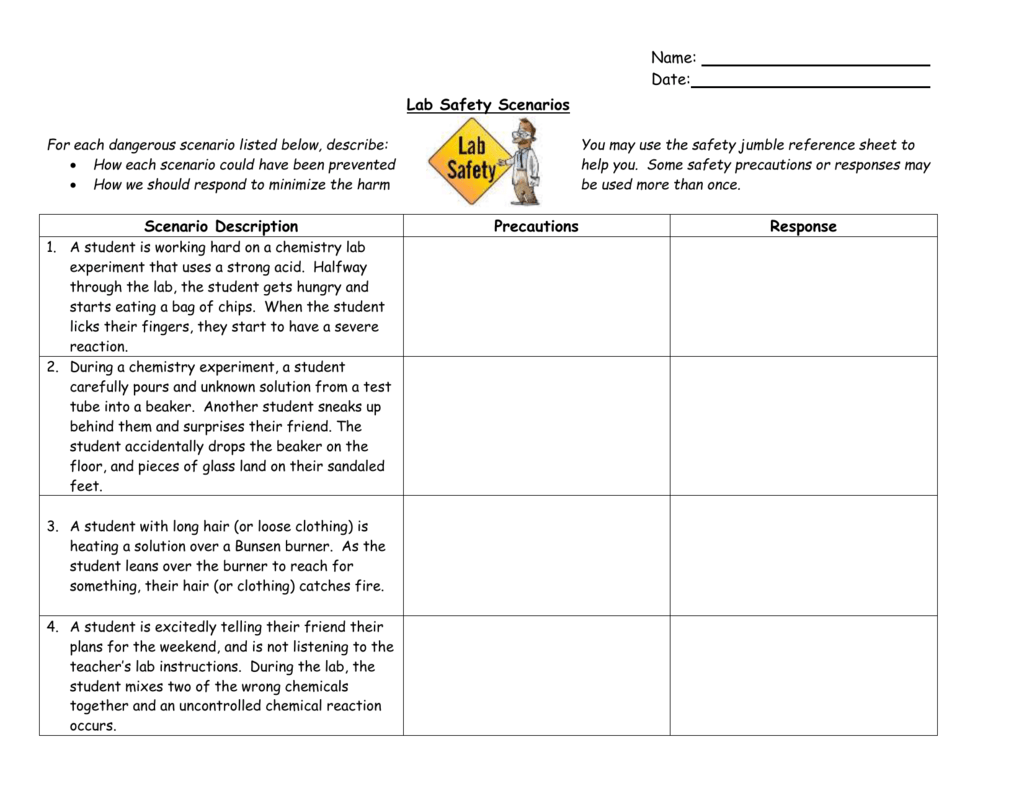
Lab Safety Worksheet Answer Key. Encouraged to help our website, in this occasion We’ll provide you with in relation to Lab Safety Worksheet Answer Key.

What about photograph previously mentioned? will be which awesome???. if you think thus, I’l t show you a few image once more below:
So, if you’d like to secure these incredible photos regarding Lab Safety Worksheet Answer Key, press save link to save these photos for your personal computer. They’re available for transfer, if you’d prefer and wish to grab it, just click save badge in the post, and it’ll be instantly saved to your laptop.} Finally if you’d like to find new and recent picture related to Lab Safety Worksheet Answer Key, please follow us on google plus or save this website, we try our best to present you regular up-date with fresh and new photos. Hope you like staying here. For most upgrades and latest information about Lab Safety Worksheet Answer Key pics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on book mark section, We try to present you up-date regularly with all new and fresh images, love your exploring, and find the ideal for you.
Here you are at our website, contentabove Lab Safety Worksheet Answer Key published . At this time we’re pleased to declare we have discovered an awfullyinteresting contentto be pointed out, namely Lab Safety Worksheet Answer Key Lots of people looking for specifics ofLab Safety Worksheet Answer Key and certainly one of them is you, is not it?